Abstract
Background: Congenital plasminogen deficiency (PLGD) caused by mutations in the PLG gene is a rare disorder associated with extravascular fibrinous deposits on mucous membranes that may impair tissue and organ function (Tefs 2006; Mehta 2008). Prometic has developed an intravenous (IV) PLG product derived from human plasma (Prometic's-PLG) as replacement therapy for treatment and prevention of extravascular fibrinous (ligneous) lesions in children and adults with PLGD. Due to the rarity of this disorder and the complexity of its clinical manifestations, randomized, blinded, controlled trials are not practical. Statistical modeling comparing historical lesion data to the occurrence of lesions while on plasminogen replacement therapy may provide evidence for efficacy.
Aims: Using historical data computer modeling to determine whether replacement therapy with Prometic's-PLG results in a reduction of expected ligneous lesions in children and adults with PLGD.
Methods: Fifteen pediatric and adult subjects aged 2 to 80 years with PLGD were enrolled in an open-label study to receive multiple IV doses of Prometic's-PLG for at least 48 weeks. Subjects were dosed every 2, 3, or 4 days based upon a pharmacokinetic (PK) profile to maintain target PLG activity trough levels > 10% (absolute) above baseline values. Extravascular ligneous lesions (visible and non-visible) were assessed at baseline, weeks 4, 8, 12, and every subsequent 12 weeks up to 48 weeks. Detailed medical histories of extravascular ligneous lesions were also obtained at baseline. A model incorporating the historical visible lesion behavior of patients and their reaction to treatment is implemented. Second, simulations studies are realized based on the same data to assess the treatment effect. Furthermore, a Bayesian methodology is also implemented. The following allows for various assessments and predictions based on the predictive distributions of the parameters obtained from a longitudinal inflated Poisson Mixed Model.
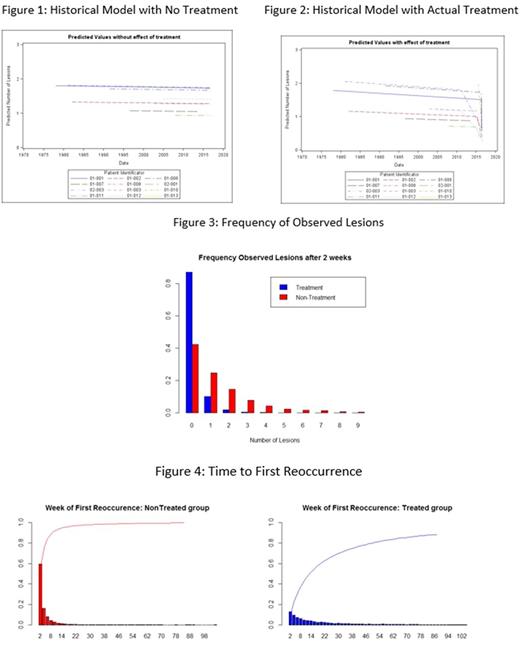
Results: Historical lesion data were available on 12 subjects at the time of the analysis. Figure 1 presents the fitted model showing the expected historical behavior of extravascular ligneous lesions in the absence of treatment with Prometic's-PLG. After 12 weeks of treatment with Prometic's-PLG, there was a statistically significant reduction (p< 0.05) in the expected number of extravascular ligneous lesions in both pediatric and adult subjects with PLGD (Figure 2).The Bayesian modeling strategy shows that the posterior distribution of the treatment effect is highly negative (very small probability of being zero or positive), which is associated with a decrease in the number of lesions and confirms the frequency model (p< 0.05). Furthermore, using the Bayesian modeling strategy, it is possible to assess the probability of having observed the reduction of lesions after the start of treatment. The model including the treatment effect shows a superiority in comparison to the model without treatment. This leads us to further believe in a departure from a usual behavior and thus a treatment effect. These models can also be used to assess the frequency of observed lesions after 2 weeks (Figure 3) under treatment and no treatment, and the time to first reoccurrence (Figure 4).
Conclusions: Historical data computer modeling highlights the significant treatment effect by Prometic's-PLG in reducing the extravascular ligneous lesions in pediatric and adult subjects with PLGD.
References:
1. Tefs K et al. Blood, 2006 Nov; 108(9):3021-6
2. Mehta R, Shapiro AD. Hemophilia, 2008 Nov; 14(6):1261-8
Parker: Prometic Biotherapeutics: Employment, Equity Ownership. Craig: Prometic Biotherapeutics: Employment, Equity Ownership. Gaido: Prometic Biotherapeutics: Employment, Equity Ownership. Albert: Prometic Biotherapeutics: Employment, Equity Ownership. Monseur: Arlenda S.A: Employment; Prometic Biotherapeutics: Consultancy. Moran: Prometic Life Sciences: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal